Abstract
Introduction: Anti-CD-19 chimeric antigen receptor-reprogrammed autologous T cells are breakthrough immunotherapies for heavily pretreated patients with aggressive B-cell lymphomas; however, across CAR-19 products, ~60% of patients do not achieve remission or relapse and unfortunately typically progress and rapidly die. Factors associated with impaired response to CAR-19 include inflammatory markers such as interferon signaling and clinical factors such as the need for bridging therapy and high pre-CAR-19 tumor burden, but cell-intrinsic driver of CAR-19 resistance remain largely undefined.
Methods: To characterize the genomic mechanisms involved in diffuse large B cell lymphoma (DLBCL) resistance to CAR-19, we interrogated whole genome sequencing (WGS) from 28 relapsed/refractory (r/r) aggressive lymphoma patients treated with axicabtagene ciloleucel (axi-cel). The median coverage was 44.8X. To increase statistical power of analyses, we included also 50 newly diagnosed DLBCL patients from the Pan-Cancer Analysis of Whole Genomes (PCAWG).
Results: As reported in other series, neither double hit cytogenetics nor MYC-BCL2 double expression associated with CAR-19 resistance, despite their negative predictive power for newly diagnosed DLBCL patients. Chapuy and LymphGen classification algorithms also demonstrated no prognostic significance. Among known mutated driver genes, only TP53 was significantly enriched in our cohort in comparison to the PCAWG cohort (p=0.002), but it did not predict poor CAR-19 outcome. Among other genes known to be involved in DLBCL pathogenesis, only mutations in NFKBIA or MYC, associated with worse PFS (p=0.04, p=0.025 respectively).
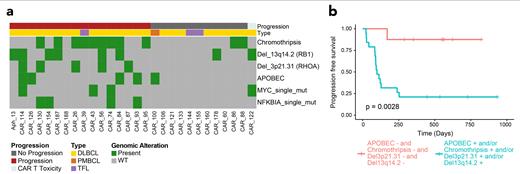
Next, we identified 12 single base substitution (SBS) mutational signatures detected in our cohort of r/r lymphomas, four of which are caused by exposure to distinct chemotherapies (Landau et al., 2020, Nat Comm). The melphalan-related signature (SBS-MM1) was identified in 4 out 5 patients who received high dose melphalan followed by autologous stem cell transplant, and 75% of patients exposed to platinum had evidence of one of the three known platinum signatures. Across different SBS signatures, only presence of APOBEC (SBS2 and SBS13) associated with worse PFS with 4/5 patients progressing (p=0.03).
We compared newly diagnosed and r/r DLBCL by GISTIC2.0 copy number variation (CNV) analysis, revealing three gene deletions significantly enriched in our r/r cohort: TP53, RHOA and RB1. Interestingly, the deletions involving RHOA and RB1 both independently predicted poor outcome (p=0.0007 and p=0.05 respectively) with 5/5 and 6/8 patients progressing respectively. The third, involving TP53 (46.4% of patients), had no prognostic impact but reflected the high-risk nature of the heavily pretreated tumors.
WGS allows detailed identification of structural variants and complex events. Indeed, we found evidence of chromothripsis, a catastrophic event in which one or more chromosomes are shattered and aberrantly reassembles generating multiple aneuploidies, in 39.3% of r/r DLBCL. This strongly associated with poor CAR-19 outcome, with 9/11 affected cases experiencing early progression (p=0.041).
Finally, reduced expression (n=3) or genomic alteration (n=3) of CD19 did not associate with poor outcome. We found one case, with durable response, containing a sub-clonal mutation in CD19 (L174V) at baseline, previously reported as associated with CAR-19 resistance. In line with recent evidence, these findings indicate that antigen-mediated tumor killing is not the only mechanism of tumor eradication, and CD19-independent resistance mechanisms predominate.
Conclusions: Leveraging the high resolution of WGS, we observed that markers of genomic complexity (chromothripsis and APOBEC) and specific genomic alterations (RHOA and RB1 deletion) associate with resistance to CAR-19 immunotherapy for aggressive B-cell lymphomas. Fifteen out of sixteen patients (93.8%) who relapsed on CAR-19 contained at least one of the described genomic alterations. Recent data demonstrate that an immunosuppressed TME leads to CAR-19 failure in patients, and animal studies show activation of host T cells by CAR-T cells. Combining these findings with these genomics findings, successful CAR-19 therapy must overcome the immune-exhausted tumor microenvironment to mobilize the host immune system and eliminate the tumor.
Jain: Takeda: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Kite/Gilead: Consultancy, Honoraria. Faramand: Novartis: Research Funding; Kite/Gilead: Research Funding. Landgren: Amgen: Research Funding; Janssen: Research Funding; Amgen: Honoraria; Celgene: Research Funding; Janssen: Other: IDMC; Janssen: Honoraria; Takeda: Other: IDMC; GSK: Honoraria. Locke: Iovance Biotherapeutics: Consultancy, Other: Scientific Advisory Role; Gerson Lehrman Group: Consultancy; Calibr: Consultancy, Other: Scientific Advisory Role; Janssen: Consultancy, Other: Scientific Advisory Role; Umoja: Consultancy, Other; Novartis: Consultancy, Other, Research Funding; Bluebird Bio: Consultancy, Other: Scientific Advisory Role; Allogene Therapeutics: Consultancy, Other: Scientific Advisory Role, Research Funding; Kite, a Gilead Company: Consultancy, Other: Scientific Advisory Role, Research Funding; Takeda: Consultancy, Other; Emerging Therapy Solutions: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Wugen: Consultancy, Other; Legend Biotech: Consultancy, Other; GammaDelta Therapeutics: Consultancy, Other: Scientific Advisory Role; Cellular Biomedicine Group: Consultancy, Other: Scientific Advisory Role; BMS/Celgene: Consultancy, Other: Scientific Advisory Role; Amgen: Consultancy, Other: Scientific Advisory Role; Moffitt Cancer Center: Patents & Royalties: field of cellular immunotherapy. Maura: Medscape: Consultancy, Honoraria; OncLive: Honoraria. Davila: Precigen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal